How to Find Number of Moles
The mole designates an extremely large number of units 602214076 1023. 4 moles Co2 to grams 4714656 grams.

Molarity M The Concentration Of A Solution As The Number Of Moles Of Solute Chemistry Education Biology Notes Chemistry
Moles are usually less than 14 inch about 6 millimeters in diameter the size of a pencil.

. The molecular mass of NH 4 2 S is 6817gmol. Also to do this you need to know the volume of the solution and how many solutes has been dissolved in the solution. The mole symbol mol is the unit of amount of substance in the International System of Units SI.
Moles consist of obvious and hidden ones. To calculate the number of. There are 60221407610 23 atoms in a mole.
2 moles Co2 to grams 2357328 grams. 1 mole of any substance contains 6022 10 23 particles. Divide the given mass 250 g by the molar mass 158032 gmol to get the moles.
The number of atoms is just a number. We can rearrange this equation to get the number of moles. You can use Molar mass of the substance alone to calculate molar mass.
Molarity is the number of moles of a substance in one litre of solution. Grams of oxygen gas 001875 moles 1600 grams. If you want to do moles to molecules conversion you need to multiply the number of moles with the Avogadros number that is 602 1023.
The moles on face are easily seen and regarded as the obvious while those on other parts of the body are called the hidden. Common moles dysplastic nevi and melanoma vary by size color shape and surface texture. The average number of moles for adults is between 10 and 40.
Find out the molar mass of the substance hint. The number of moles of KMnO4 will be 0158. For one mole the atomic or molecular mass will be the same as the weight.
Moles can be brown tan black blue red or pink. The official symbol for molarity is c concentration but many people use the old symbol M. 5 moles Co2 to grams 589332 grams.
Formula to calculate number of moles weightatomic mass of the element 8040 2 Hence number of moles in calcium is 2 In our below online number of moles calculator enter the weight and select the element from the drop-down menu. The molar mass of KMnO4 is 158032 gmol. Moles appear on our skin from sun exposure ultraviolet radiation or we are born with them.
The location of moles is greatly related to peoples fortune. Divide 2 by 6817 and you have 00293 moles of NH 4 2 S. One mole of a substance is equal to 6022 10²³ units of that substance such as atoms molecules or ions.
Learn about moles nevi what causes them whether they put you at increased risk of skin cancer and how to check a mole for signs of skin cancer. They may have hair growing from them. 3 moles Co2 to grams 3535992 grams.
7 moles Co2 to grams 8250648 grams. Most moles are oval or round. They can be smooth wrinkled flat or raised.
The result is the number of moles in your element or compound. Convert the moles of substance to desired units of measure. Find the molarity by calculating the number of moles of the solute dissolved in liters of a solution.
Number of atoms moles Avogadros number. M nV where n is the number of moles and V is the volume in litres. To answer your example question there are 313 10 23 atoms in 3280 grams of copper.
Mole also spelled mol in chemistry a standard scientific unit for measuring large quantities of very small entities such as atoms molecules or other specified particles. Rearrange the formula to find Avogadros constant. In 1 kg-moles of any substance there exists exactly 103.
10 moles Co2 to grams 1178664 grams. Moles of oxygen 00125 moles ozone 3 moles oxygen2 moles ozone. Sample Molarity Calculation Calculate the molarity of a solution prepared by dissolving 237 grams of KMnO 4 into enough water to make 750 mL of solution.
The reason is that one mole of the substance contains the amount of moles that are exactly in 12 grams of the carbon-12. Advice updates and vaccine options Find out about COVID-19 COVID-19 vaccines. Using a calculator divide the number of grams by the molar mass.
Solving for this you get 001875 moles of oxygen gas. Finally convert the number of moles of oxygen gas into grams for the answer. The list below summarizes some differences between moles and cancer.
The number 6022 10²³ is known as Avogadros number or Avogadros constant. Write the half-reactions that take place at the anode and at the cathode. N n N A N number of particles in the substance n amount of substance in moles mol N A Avogardro Number 6022 10 23 particles mol-1.
6 moles Co2 to grams 7071984 grams. 8 moles Co2 to grams 9429312 grams. Another important difference is that a common mole or dysplastic nevus will not return after it is removed by a full excisional biopsy from the skin but melanoma sometimes grows back.
How many atoms in a mole. As the left-hand side equals the right-hand side the Avogadros. Definition of Number of Moles.
Depending on what the substance is an elementary entity may be an. Mass of MnO 2 95g. We know the atomic mass of calcium is 40.
Notice the number of moles is used from Step 2 to start the calculation from moles to atoms. N n 6022 10 23. Steps 2 and 3 can be combined.
Mass of one mole MnO 2 8694g. Therefore it is dimensionless ie it does not any units. The mole is defined as containing exactly 6022 140 76 10 23 elementary entities.
Number of moles 1092 mol. Number of moles 95 8694. To work it out find the atomic or molecular mass of your substance and multiply it by the number of moles you have.
How to find moles in the solution is to calculate how many molecules the solution contains. Moles can cause significant problems in landscape or garden areas especially in turf. Also you can make use of the free grams to moles calculator to calculate the number of moles instantly and precisely.
The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. Calculate the number of moles of electrons that were transferred. Determine the number of moles in 95g of MnO 2.
So on the left-hand side we have no units moles which can be expressed as mol-1. Number of moles formula is. Number of moles Mass of substance Mass of one mole.
Because mole damage can be unsightly makes lawn maintenance difficult and destroys valuable plants the number of moles that can be tolerated is usually quite low sometimes even zero. Generally the hidden moles such as in the eyebrows beard hair etc are auspicious and the obvious are. TextNumber of Molecules 5 602 1023 textNumber of Molecules 301 10.
For example imagine you have 2 g of NH 4 2 S and you want to convert it to moles. 052 mol of Cu 6022 x 10 23 atoms 1 mol of Cu 313 x 10 23 molecules. Use the mole ratio to find how many moles of oxygen are produced by 00125 moles of ozone.
The General Conference on Weights and Measures defined the mole as this number for the International System of Units SI effective. Find the number of moles. It refers to a huge number that we use.
Furthermore you also need to know the molar mass of the solute. The concept of the mole can be used to convert between mass and number of particles. How many moles are there in 1kg.
O C and 1 atm that is produced during this time. Although the number of moles varies from person to person fair-skinned people generally have more moles due to lower amounts of melanin in their skin. If we have 5 moles of calcium then the number of molecules in it will be as follows.
Convert 250 grams of KMnO4 to moles. 9 moles Co2 to grams 10607976 grams. But moles come in different colors shapes and sizes.
Calculate the number of moles of substance that was producedconsumed at the electrode. 6022 10 23 is known as the Avogadro Number or Avogadro Constant and is given the symbol N A 1.

Mole Ratios Mole Ratio Relationship

Mole Ratio Easy Science 10th Grade Science Chemistry Notes Easy Science
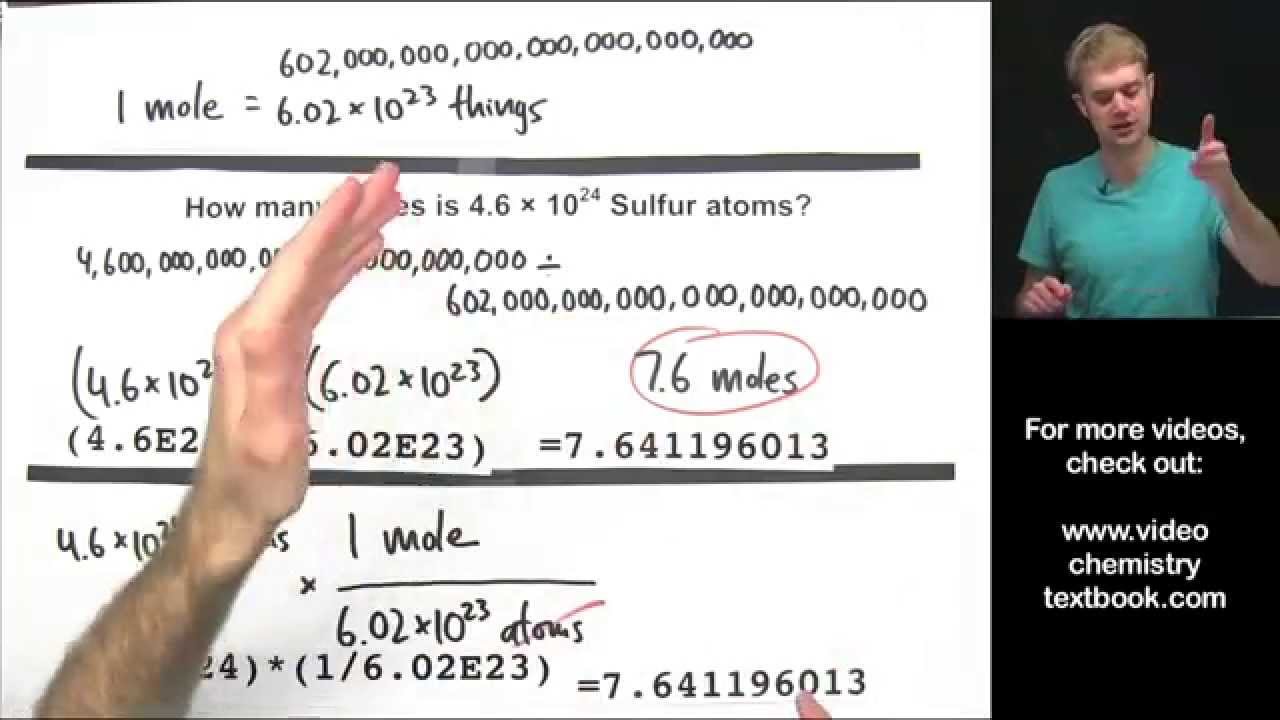
Converting Between Moles Atoms And Molecules Teaching Chemistry Chemistry Textbook High School Chemistry

No comments for "How to Find Number of Moles"
Post a Comment